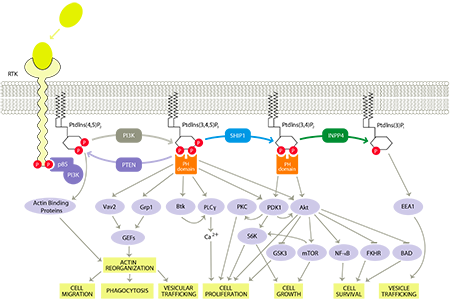
SHIP1 and the PI3K Pathway – Highlighting the Role of SHIP1
Click graphic for enlarged view.
The PI3K pathway is a cellular signaling pathway that has been linked to a diverse group of cellular functions and biological processes such as cell activation and migration, which are related to inflammation, and cell growth, proliferation and survival, which are related to cancer. As a result, the PI3K pathway is heavily researched by the academic community as well as pharmaceutical and biotechnology companies in the areas of immune disorders and cancer.
If the PI3K pathway is overactive, immune cells can produce an abundance of pro-inflammatory signaling molecules and migrate to and concentrate in tissues, resulting in excessive or chronic inflammation. SHIP1 is predominantly present in immune cells and when activated, SHIP1 redirects signaling in immune cells to reduce their activation and migration, thereby reducing inflammation while still allowing these cells to maintain cell growth and survival, taking advantage of the natural modulation of the PI3K pathway. Consequently, drugs that activate SHIP1 can reduce the function and migration of immune cells and have an anti-inflammatory effect.
Our approach also targets a unique activation site in SHIP1 called the C2 binding domain. We have demonstrated that targeting the C2 binding domain does not significantly activate or inhibit other enzymes, imparting target selectivity and further limiting potential off-target toxicities. Therefore, SHIP1 activators target immune cells to cause an anti-inflammatory effect while minimizing effects in other tissues.
We believe AQX-1125 is the only SHIP1 activator currently in clinical trials and that no SHIP1 activator has yet received marketing approval as a treatment for disease in humans.
Our scientific founders, based at the University of British Columbia, were the first to discover SHIP1 and show that small molecules could activate it, thereby making it a potential target for a new class of anti-inflammatory drugs. Additionally, academic scientists have shown that certain immune cell cancers have suppressed levels of SHIP1, making such cancers also potential targets for SHIP1 activators.