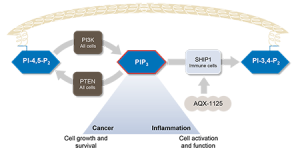
The SHIP1 Pathway – Highlighting the Role of AQX-1125
Click graphic for enlarged view.
AQX-1125 is our lead product candidate and has generated positive clinical data from three completed clinical trials, including two proof-of-concept trials, one in COPD and one in allergic asthma, demonstrating a favorable safety profile and anti-inflammatory activity. Overall, more than 100 subjects have received AQX-1125. Importantly, our clinical trial results were consistent with the drug-like properties and anti-inflammatory activities demonstrated in our preclinical studies. AQX-1125 is a once daily oral capsule with many desirable drug-like properties. We are currently investigating AQX-1125 in two Phase 2 clinical trials, one in COPD and one in BPS/IC.
Based on our three completed clinical trials, we have demonstrated that AQX-1125:
- has desirable pharmacokinetic, absorption and excretion properties that make it suitable for once daily oral administration;
- is generally well tolerated, exhibiting mild to moderate adverse events primarily related to gastrointestinal upset that resolve without treatment or long-term effects and are reduced by taking the drug candidate with food; and
- has anti-inflammatory properties consistent with those exhibited in preclinical studies and exhibited activity in two trials using two distinct inflammatory challenges.
AQX-1125 is an activator of SHIP1, which controls the PI3K cellular signaling pathway. If the PI3K pathway is overactive, immune cells can produce an abundance of pro-inflammatory signaling molecules and migrate to and concentrate in tissues, resulting in excessive or chronic inflammation. SHIP1 is predominantly expressed in cells derived from bone marrow tissues, which are mainly immune cells. Therefore drugs that activate SHIP1 can reduce the function and migration of immune cells and have an anti-inflammatory effect. By controlling the PI3K pathway, AQX-1125 reduces immune cell function and migration by targeting a mechanism that has evolved in nature to maintain homeostasis of the immune system.
AQX-1125 has demonstrated compelling preclinical activity in a broad range of relevant inflammatory studies including preclinical models of COPD, asthma, pulmonary fibrosis, BPS/IC and inflammatory bowel disease (IBD). In these studies we have seen a meaningful reduction in the relevant immune cells that are the cells that cause inflammation, such as neutrophils, eosinophils and macrophages, and a reduction in the symptoms of inflammation, such as pain and swelling. The activity, efficacy and potency seen with AQX-1125 in most preclinical studies compare favorably to published results with corticosteroids. In addition, AQX-1125 demonstrated compelling activity in the smoke airway inflammation and Bleomycin Fibrosis models, which are known to be steroid refractory, or in other words, do not respond to corticosteroids. We believe this broad anti-inflammatory profile is not typical amongst drugs in development and supports the therapeutic potential for AQX-1125.
In addition to demonstrating strong in vitro and in vivo activity, AQX-1125 was also selected as a lead candidate based on its many desirable drug-like properties. The drug candidate is highly water soluble and does not require complex formulation for oral administration. AQX-1125 has low plasma protein binding, is not metabolized and is excreted unmetabolized in both urine and feces. After oral or intravenous dosing, AQX-1125 reaches high concentrations in respiratory, urinary and gastrointestinal tracts, all of which have mucosal surfaces of therapeutic interest. In humans, AQX-1125 has shown pharmacokinetic properties suitable for once-a-day dosing. In addition, the absorption of the drug candidate is equivalent whether taken with or without food.